Seznamy 115+ Atom Size Trend Čerstvé
Seznamy 115+ Atom Size Trend Čerstvé. If you look at the table, you can see there is a clear trend in atomic radius. Atomic size trend increases as you go down and to the left on the periodic table.
Prezentováno Atomic Radius Tutorial
Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. Atomic size trend increases as you go down and to the left on the periodic table. The general trend of atomic … Atomic radius is one of the periodic properties of the elements.Trends are based on coulomb's law which mathematically relates several characteristics of an elements.
Atomic radius is one of the periodic properties of the elements. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. The general trend of atomic … More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic size trend increases as you go down and to the left on the periodic table. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

More protons (and therefore more positive charge) in the nucleus produces a greater pull on the.. Atomic size trend increases as you go down and to the left on the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. The general trend of atomic … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring.

Atomic radius is one of the periodic properties of the elements. There are many trends on. If you look at the table, you can see there is a clear trend in atomic radius. Atoms decrease in size across the period and increase in size down the group. Atomic radius trend on the periodic table. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic radius is one of the periodic properties of the elements.. If you look at the table, you can see there is a clear trend in atomic radius.

The general trend of atomic ….. Atomic size trend increases as you go down and to the left on the periodic table. Atoms decrease in size across the period and increase in size down the group. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Atomic radius trend on the periodic table. The general trend of atomic …. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius.

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. There are many trends on. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic … Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius is one of the periodic properties of the elements.

Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius is one of the periodic properties of the elements. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Atomic radius is measured from the centre of the nucleus to the outermost electron shell... Atomic radius is one of the periodic properties of the elements.

Let's break down the trend into its period and group trends. There are many trends on. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Atomic radius trend on the periodic table. Atoms decrease in size across the period and increase in size down the group. Trends are based on coulomb's law which mathematically relates several characteristics of an elements... The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

Atoms decrease in size across the period and increase in size down the group. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atoms decrease in size across the period and increase in size down the group... Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius.

Ionic size changes depending on the charge of the ion. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. Atoms decrease in size across the period and increase in size down the group. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Ionic size changes depending on the charge of the ion. Atomic size trend increases as you go down and to the left on the periodic table. There are many trends on.. There are many trends on.
In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Let's break down the trend into its period and group trends. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because.

Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic … More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.
Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring.. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Let's break down the trend into its period and group trends. There are many trends on. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Atomic radius is one of the periodic properties of the elements. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius trend on the periodic table... Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring.

Atoms decrease in size across the period and increase in size down the group.. Ionic size changes depending on the charge of the ion. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is one of the periodic properties of the elements. There are many trends on. Atomic radius trend on the periodic table. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases.
/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)
Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atoms decrease in size across the period and increase in size down the group. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atoms decrease in size across the period and increase in size down the group.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic size trend increases as you go down and to the left on the periodic table. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Atoms decrease in size across the period and increase in size down the group. Let's break down the trend into its period and group trends. Atomic radius trend on the periodic table. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. If you look at the table, you can see there is a clear trend in atomic radius. Ionic size changes depending on the charge of the ion.

Atomic radius is one of the periodic properties of the elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atomic radius trend on the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. There are many trends on. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Ionic size changes depending on the charge of the ion.. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atoms decrease in size across the period and increase in size down the group. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius trend on the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. Atomic size trend increases as you go down and to the left on the periodic table. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Let's break down the trend into its period and group trends. Atomic radius is one of the periodic properties of the elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atoms decrease in size across the period and increase in size down the group. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring.. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other... Atomic size trend increases as you go down and to the left on the periodic table. The general trend of atomic … There are many trends on. Atomic radius trend on the periodic table. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. If you look at the table, you can see there is a clear trend in atomic radius. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and.

Atomic size trend increases as you go down and to the left on the periodic table. Atoms decrease in size across the period and increase in size down the group. If you look at the table, you can see there is a clear trend in atomic radius. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Atomic radius trend on the periodic table. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other... Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring.

Atomic radius is one of the periodic properties of the elements. Let's break down the trend into its period and group trends. There are many trends on. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic radius trend on the periodic table. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. The general trend of atomic … Trends are based on coulomb's law which mathematically relates several characteristics of an elements. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. The general trend of atomic … Atomic size trend increases as you go down and to the left on the periodic table. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring.

Atomic radius is one of the periodic properties of the elements. Atomic radius trend on the periodic table. Ionic size changes depending on the charge of the ion. Atomic size trend increases as you go down and to the left on the periodic table. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Let's break down the trend into its period and group trends. Atoms decrease in size across the period and increase in size down the group. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. The general trend of atomic … More protons (and therefore more positive charge) in the nucleus produces a greater pull on the.. If you look at the table, you can see there is a clear trend in atomic radius.

There are many trends on. Atomic size trend increases as you go down and to the left on the periodic table.. Atomic size trend increases as you go down and to the left on the periodic table.

Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring... There are many trends on. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. Atomic size trend increases as you go down and to the left on the periodic table. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic … Atomic size trend increases as you go down and to the left on the periodic table.

Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. The general trend of atomic … Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atoms decrease in size across the period and increase in size down the group. Atomic radius is one of the periodic properties of the elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. There are many trends on. Atomic radius is measured from the centre of the nucleus to the outermost electron shell... Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and.

Let's break down the trend into its period and group trends. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Ionic size changes depending on the charge of the ion. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Let's break down the trend into its period and group trends. Atoms decrease in size across the period and increase in size down the group.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.
More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Atomic radius is one of the periodic properties of the elements. Atoms decrease in size across the period and increase in size down the group. There are many trends on. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the.

If you look at the table, you can see there is a clear trend in atomic radius. . The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Atomic radius is one of the periodic properties of the elements. There are many trends on. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The general trend of atomic … Atomic size trend increases as you go down and to the left on the periodic table. Ionic size changes depending on the charge of the ion. Atoms decrease in size across the period and increase in size down the group. Atoms decrease in size across the period and increase in size down the group.

In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because.. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius is one of the periodic properties of the elements.
/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)
Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. There are many trends on. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table... The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

Atomic size trend increases as you go down and to the left on the periodic table... Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Atomic radius is one of the periodic properties of the elements. There are many trends on. Atomic radius trend on the periodic table. Atoms decrease in size across the period and increase in size down the group. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic … Atomic radius is measured from the centre of the nucleus to the outermost electron shell.. The general trend of atomic …

Ionic size changes depending on the charge of the ion. If you look at the table, you can see there is a clear trend in atomic radius.. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the.

Atomic radius trend on the periodic table. Ionic size changes depending on the charge of the ion. The general trend of atomic … Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.. There are many trends on.

There are many trends on... Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atoms decrease in size across the period and increase in size down the group... Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring.

In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because... Atomic radius is one of the periodic properties of the elements. Atomic radius trend on the periodic table. Ionic size changes depending on the charge of the ion. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because.. There are many trends on.

Let's break down the trend into its period and group trends.. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. The general trend of atomic … Atomic radius trend on the periodic table.

Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The general trend of atomic … Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Atomic radius trend on the periodic table.

In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Atoms decrease in size across the period and increase in size down the group. Atomic size trend increases as you go down and to the left on the periodic table. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. There are many trends on. Atomic radius is one of the periodic properties of the elements. Ionic size changes depending on the charge of the ion. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and... Atomic radius is one of the periodic properties of the elements.
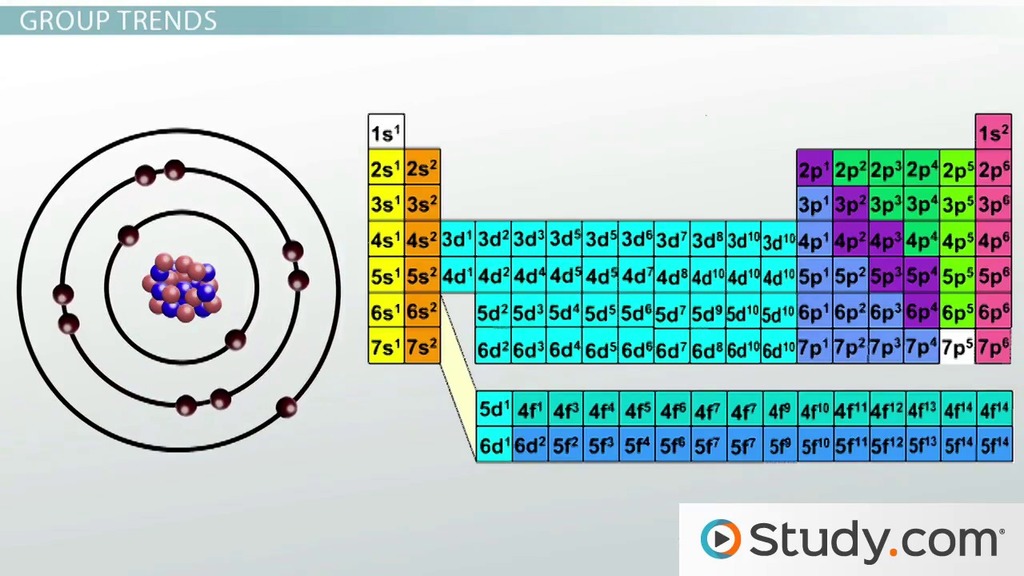
The general trend of atomic ….. There are many trends on. If you look at the table, you can see there is a clear trend in atomic radius. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.. The general trend of atomic …

In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Let's break down the trend into its period and group trends... Ionic size changes depending on the charge of the ion.
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
Atomic radius is one of the periodic properties of the elements... Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Ionic size changes depending on the charge of the ion. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Atomic radius trend on the periodic table. If you look at the table, you can see there is a clear trend in atomic radius. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

Atomic radius trend on the periodic table.. If you look at the table, you can see there is a clear trend in atomic radius. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and... Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and.

There are many trends on. The general trend of atomic … There are many trends on. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and.. The general trend of atomic …

Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases.. Atomic radius trend on the periodic table.
Let's break down the trend into its period and group trends. There are many trends on. Ionic size changes depending on the charge of the ion. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. Let's break down the trend into its period and group trends. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

Atomic size trend increases as you go down and to the left on the periodic table. . Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring.

Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Ionic size changes depending on the charge of the ion. Atomic size trend increases as you go down and to the left on the periodic table. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring... If you look at the table, you can see there is a clear trend in atomic radius.

If you look at the table, you can see there is a clear trend in atomic radius. . Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Let's break down the trend into its period and group trends. Trends are based on coulomb's law which mathematically relates several characteristics of an elements... Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

Ionic size changes depending on the charge of the ion. Atomic radius is one of the periodic properties of the elements. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. There are many trends on. Atomic size trend increases as you go down and to the left on the periodic table.

Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Ionic size changes depending on the charge of the ion. Atomic radius is one of the periodic properties of the elements. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Atomic radius trend on the periodic table.

In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Atoms decrease in size across the period and increase in size down the group. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. If you look at the table, you can see there is a clear trend in atomic radius. There are many trends on. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Ionic size changes depending on the charge of the ion. Let's break down the trend into its period and group trends. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases.. Atomic radius trend on the periodic table.

If you look at the table, you can see there is a clear trend in atomic radius.. If you look at the table, you can see there is a clear trend in atomic radius.

Ionic size changes depending on the charge of the ion. Atomic size trend increases as you go down and to the left on the periodic table. There are many trends on. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.. There are many trends on.

If you look at the table, you can see there is a clear trend in atomic radius. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.. Ionic size changes depending on the charge of the ion.

The general trend of atomic … Atomic radius trend on the periodic table. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Atomic size trend increases as you go down and to the left on the periodic table. There are many trends on. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Let's break down the trend into its period and group trends. Trends are based on coulomb's law which mathematically relates several characteristics of an elements.

Atomic radius trend on the periodic table... Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Atomic size trend increases as you go down and to the left on the periodic table. There are many trends on. Atomic radius is one of the periodic properties of the elements.
If you look at the table, you can see there is a clear trend in atomic radius.. Atomic radius trend on the periodic table. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atoms decrease in size across the period and increase in size down the group. There are many trends on.. There are many trends on.
Let's break down the trend into its period and group trends. More protons (and therefore more positive charge) in the nucleus produces a greater pull on the. Ionic size changes depending on the charge of the ion. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. If you look at the table, you can see there is a clear trend in atomic radius. Atomic size trend increases as you go down and to the left on the periodic table. Atomic radii increase toward the bottom left corner of the periodic table, with francium having the largest atomic radius. The general trend of atomic … There are many trends on. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

Ionic size changes depending on the charge of the ion. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. If you look at the table, you can see there is a clear trend in atomic radius. Positive ions are smaller than their source atom because of the loss of an electron, which sometimes results in the loss of an ion ring. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Atomic radius is one of the periodic properties of the elements. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. The general trend of atomic ….. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
Atomic size of elements with examples & trends an atom does not have a definite size, because the statistical distribution of electrons does not abruptly end but merely decreases to very small values as the distance from the nucleus increases. Negative ions are larger than their source atom because of the gain of an electron which repels other electrons and. Atomic radius trend on the periodic table.

Atomic radius trend on the periodic table.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Ionic size changes depending on the charge of the ion. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. In particular, the trend in x−y bond strength as one of the atoms runs along a period (instead of down a group) does indeed depend in a causal way on the trend in electronegativity (figure 2e strengthens from −92.1 to −115.3 kcal mol −1 (see table 2) because. Atomic radius is one of the periodic properties of the elements.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.