Sbírka Atom Size Trend Periodic Table Čerstvý
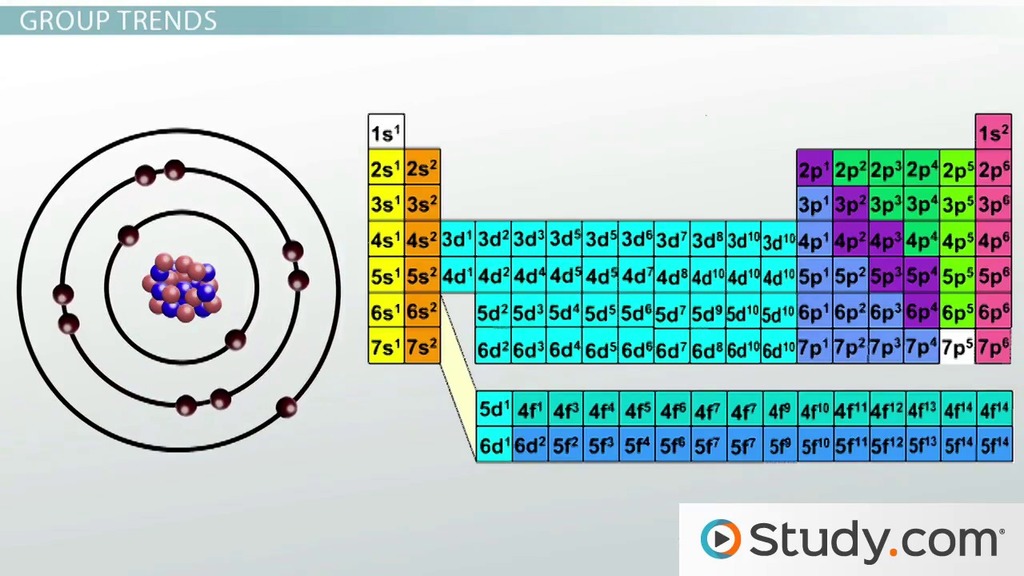
Sbírka Atom Size Trend Periodic Table Čerstvý. 1) across a row, and 2) up and down a column. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.
Tady Labeled Periodic Table Science Trends
The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Yeah, he is even smaller than hydrogen, h, which is 53 pm. The smallest atom on the periodic table is helium, he, and has a radius of 31 pm. While moving down in the group (from top to bottom), the atomic radius increases. Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom.As you move down a group in the periodic table, the covalent radius increases.
All our trends describe the trend in two directions on the periodic table: The smallest atom on the periodic table is helium, he, and has a radius of 31 pm. Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom. As you move down a group in the periodic table, the covalent radius increases. 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table. When o gains two electrons to form o2− the.

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period... In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. That would be cesium, cs, which comes in with a radius of 343 pm. 13.04.2014 · an ion's size, compared to its parent atom, depends on whether it gains or loses electrons.. Atomic radius varies the lower an atom appears on periodic table.

For example, as we move from left to right in a period, atomic size decreases. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 15.03.2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic radius can be linked to core …. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases.

Atomic radius can be linked to core … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 15.03.2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic radius varies the lower an atom appears on periodic table. The smallest atom on the periodic table is helium, he, and has a radius of 31 pm. If you look at the table, you can see there is a clear trend in atomic radius. 2 see answers advertisement … Which atom is the largest?

01.11.2021 · atomic radius trend in periodic table. All our trends describe the trend in two directions on the periodic table: Which atom is the largest? Atomic radius is one of the periodic properties of the elements. 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table. As you move down a group in the periodic table, the covalent radius increases. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. 1) across a row, and 2) up and down a column. For example if na loses one electron to form n a+, the latter is smaller than the na atom. 06.11.2014 · atomic radius trend on the periodic table. Thus, we can say that elements having similar electronic configuration have similar properties. Atomic radius gets smaller the lower an atom appears on the periodic table.

While moving down in the group (from top to bottom), the atomic radius increases. Atomic radius can be linked to core … Here are the important ones for us. 01.11.2021 · atomic radius trend in periodic table. Yeah, he is even smaller than hydrogen, h, which is 53 pm.. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity... Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom. 2 see answers advertisement … 1) across a row, and 2) up and down a column. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic radius stays the same in a column of the period table c. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is one of the periodic properties of the elements... Here are the important ones for us.

Yeah, he is even smaller than hydrogen, h, which is 53 pm. While moving down in the group (from top to bottom), the atomic radius increases. 15.03.2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.. Atomic radius is one of the periodic properties of the elements.

Which atom is the largest? Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom. Atomic radius gets bigger the lower an atom appeares on the periodic table b. While moving down in the group (from top to bottom), the atomic radius increases. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. 13.04.2014 · an ion's size, compared to its parent atom, depends on whether it gains or loses electrons. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 15.03.2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Atomic radius stays the same in a column of the period table c. Atomic radius varies the lower an atom appears on periodic table. For example if na loses one electron to form n a+, the latter is smaller than the na atom.

In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases... .. Atomic radius can be linked to core …

23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table.. Although we find some exceptions which do not follow these periodic table trends. Atomic radius stays the same in a column of the period table c. Here are the important ones for us. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases.

Atomic radius can be linked to core … Which atom is the largest? 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table. Although we find some exceptions which do not follow these periodic table trends. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. Yeah, he is even smaller than hydrogen, h, which is 53 pm. 06.11.2014 · atomic radius trend on the periodic table. Atomic radius stays the same in a column of the period table c. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table.

Thus, we can say that elements having similar electronic configuration have similar properties. As you move down a group in the periodic table, the covalent radius increases. 15.03.2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. If you look at the table, you can see there is a clear trend in atomic radius... As we move across the period (from left to right), the atomic radius or atomic size of elements decreases.

In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius gets bigger the lower an atom appeares on the periodic table b. If you look at the table, you can see there is a clear trend in atomic radius. 01.11.2021 · atomic radius trend in periodic table. Atomic radius stays the same in a column of the period table c. Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom... For example, as we move from left to right in a period, atomic size decreases.

If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius stays the same in a column of the period table c. Thus, we can say that elements having similar electronic configuration have similar properties. Although we find some exceptions which do not follow these periodic table trends.. Atomic radius gets smaller the lower an atom appears on the periodic table.

Yeah, he is even smaller than hydrogen, h, which is 53 pm. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Although we find some exceptions which do not follow these periodic table trends. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius gets bigger the lower an atom appeares on the periodic table b. 06.11.2014 · atomic radius trend on the periodic table. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. All our trends describe the trend in two directions on the periodic table: That would be cesium, cs, which comes in with a radius of 343 pm. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 15.03.2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table.. Atomic radius varies the lower an atom appears on periodic table.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. As we move across the period (from left to right), the atomic radius or atomic size of elements decreases. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. 01.11.2021 · atomic radius trend in periodic table. While moving down in the group (from top to bottom), the atomic radius increases. 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table.
.PNG)
That would be cesium, cs, which comes in with a radius of 343 pm. Here are the important ones for us. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Although we find some exceptions which do not follow these periodic table trends. 26.03.2019 · what trend is seen in atom size, going down the periodic table? Atomic radius varies the lower an atom appears on periodic table. This is because of the screening effect of the filled inner electron levels. As we move across the period (from left to right), the atomic radius or atomic size of elements decreases.. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 1) across a row, and 2) up and down a column.. Here are the important ones for us.

The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. The smallest atom on the periodic table is helium, he, and has a radius of 31 pm. That would be cesium, cs, which comes in with a radius of 343 pm. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Atomic radius can be linked to core … Atomic radius varies the lower an atom appears on periodic table. 26.03.2019 · what trend is seen in atom size, going down the periodic table? Yeah, he is even smaller than hydrogen, h, which is 53 pm. Which atom is the largest? When o gains two electrons to form o2− the... Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom.

While moving down in the group (from top to bottom), the atomic radius increases. Atomic radius varies the lower an atom appears on periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. For example, as we move from left to right in a period, atomic size decreases. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius stays the same in a column of the period table c. Atomic radius gets bigger the lower an atom appeares on the periodic table b. 2 see answers advertisement … Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.
/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)
Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom. Although we find some exceptions which do not follow these periodic table trends. That would be cesium, cs, which comes in with a radius of 343 pm. Atomic radius gets bigger the lower an atom appeares on the periodic table b.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

For example, as we move from left to right in a period, atomic size decreases... 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table. For example if na loses one electron to form n a+, the latter is smaller than the na atom. 01.11.2021 · atomic radius trend in periodic table. As we move across the period (from left to right), the atomic radius or atomic size of elements decreases. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius stays the same in a column of the period table c.

1) across a row, and 2) up and down a column... 26.03.2019 · what trend is seen in atom size, going down the periodic table? 15.03.2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. That would be cesium, cs, which comes in with a radius of 343 pm.

As you move down a group in the periodic table, the covalent radius increases.. If you look at the table, you can see there is a clear trend in atomic radius. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other. Here are the important ones for us. While moving down in the group (from top to bottom), the atomic radius increases. Which atom is the largest? In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Atomic radius varies the lower an atom appears on periodic table. For example if na loses one electron to form n a+, the latter is smaller than the na atom. Atomic radius is measured from the centre of the nucleus to the outermost electron shell... This is because of the screening effect of the filled inner electron levels.

06.11.2014 · atomic radius trend on the periodic table. . 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table.

The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Although we find some exceptions which do not follow these periodic table trends. Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. 26.03.2019 · what trend is seen in atom size, going down the periodic table? As you move down a group in the periodic table, the covalent radius increases.

Trends are based on coulomb's law which mathematically relates several characteristics of an elements... . Atomic radius is one of the periodic properties of the elements.

While moving down in the group (from top to bottom), the atomic radius increases. This is because of the screening effect of the filled inner electron levels. For example, as we move from left to right in a period, atomic size decreases.

Yeah, he is even smaller than hydrogen, h, which is 53 pm. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. 2 see answers advertisement … Atomic radius stays the same in a column of the period table c. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 01.11.2021 · atomic radius trend in periodic table. When o gains two electrons to form o2− the. As we move across the period (from left to right), the atomic radius or atomic size of elements decreases... Thus, we can say that elements having similar electronic configuration have similar properties.

All our trends describe the trend in two directions on the periodic table:. 06.11.2014 · atomic radius trend on the periodic table... Thus, we can say that elements having similar electronic configuration have similar properties.

The smallest atom on the periodic table is helium, he, and has a radius of 31 pm... Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom. Here are the important ones for us. When o gains two electrons to form o2− the. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius gets smaller the lower an atom appears on the periodic table.

In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. All our trends describe the trend in two directions on the periodic table: Yeah, he is even smaller than hydrogen, h, which is 53 pm. That would be cesium, cs, which comes in with a radius of 343 pm. 15.03.2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. When o gains two electrons to form o2− the. 06.11.2014 · atomic radius trend on the periodic table. Which atom is the largest? This is because of the screening effect of the filled inner electron levels.. 06.11.2014 · atomic radius trend on the periodic table.

Atomic radius can be linked to core … Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius stays the same in a column of the period table c. Although we find some exceptions which do not follow these periodic table trends. Atomic radius varies the lower an atom appears on periodic table. For example if na loses one electron to form n a+, the latter is smaller than the na atom. 1) across a row, and 2) up and down a column. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. 2 see answers advertisement … Atomic radius is one of the periodic properties of the elements. If you look at the table, you can see there is a clear trend in atomic radius. This is because of the screening effect of the filled inner electron levels.

For example, as we move from left to right in a period, atomic size decreases. As you move down a group in the periodic table, the covalent radius increases. 06.11.2014 · atomic radius trend on the periodic table. For example if na loses one electron to form n a+, the latter is smaller than the na atom. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. Thus, we can say that elements having similar electronic configuration have similar properties. When o gains two electrons to form o2− the. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.

As we move across the period (from left to right), the atomic radius or atomic size of elements decreases. Atomic radius can be linked to core … With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Yeah, he is even smaller than hydrogen, h, which is 53 pm. Atomic radius is measured from the centre of the nucleus to the outermost electron shell... Trends are based on coulomb's law which mathematically relates several characteristics of an elements.
.PNG)
All our trends describe the trend in two directions on the periodic table:. Which atom is the largest?

Here are the important ones for us.. . Atomic radius gets smaller the lower an atom appears on the periodic table.

For example, as we move from left to right in a period, atomic size decreases... The smallest atom on the periodic table is helium, he, and has a radius of 31 pm. 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table. As we move across the period (from left to right), the atomic radius or atomic size of elements decreases... Atomic radius stays the same in a column of the period table c.

13.04.2014 · an ion's size, compared to its parent atom, depends on whether it gains or loses electrons. As you move down a group in the periodic table, the covalent radius increases. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. 26.03.2019 · what trend is seen in atom size, going down the periodic table? 15.03.2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Thus, we can say that elements having similar electronic configuration have similar properties. When o gains two electrons to form o2− the. 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table.. This is because of the screening effect of the filled inner electron levels.
Atomic radius varies the lower an atom appears on periodic table. While moving down in the group (from top to bottom), the atomic radius increases. Here are the important ones for us. 06.11.2014 · atomic radius trend on the periodic table. This is because of the screening effect of the filled inner electron levels. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. 26.03.2019 · what trend is seen in atom size, going down the periodic table? Atomic radius gets smaller the lower an atom appears on the periodic table.. With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table.

Which atom is the largest? Which atom is the largest? Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Yeah, he is even smaller than hydrogen, h, which is 53 pm.. Atomic radius can be linked to core …
As you move down a group in the periodic table, the covalent radius increases. Atomic radius gets bigger the lower an atom appeares on the periodic table b. As we move across the period (from left to right), the atomic radius or atomic size of elements decreases. The smallest atom on the periodic table is helium, he, and has a radius of 31 pm. All our trends describe the trend in two directions on the periodic table: Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. 15.03.2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. 13.04.2014 · an ion's size, compared to its parent atom, depends on whether it gains or loses electrons. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases.
Atomic radius gets smaller the lower an atom appears on the periodic table. Atomic radius gets bigger the lower an atom appeares on the periodic table b. Which atom is the largest? That would be cesium, cs, which comes in with a radius of 343 pm. Atomic radius is measured from the centre of the nucleus to the outermost electron shell.

All our trends describe the trend in two directions on the periodic table: Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom. As we move across the period (from left to right), the atomic radius or atomic size of elements decreases. All our trends describe the trend in two directions on the periodic table: The smallest atom on the periodic table is helium, he, and has a radius of 31 pm. Which atom is the largest? Atomic radius varies the lower an atom appears on periodic table... Although we find some exceptions which do not follow these periodic table trends.
:max_bytes(150000):strip_icc()/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table... The smallest atom on the periodic table is helium, he, and has a radius of 31 pm. For example if na loses one electron to form n a+, the latter is smaller than the na atom. This is because of the screening effect of the filled inner electron levels.
26.03.2019 · what trend is seen in atom size, going down the periodic table? If you look at the table, you can see there is a clear trend in atomic radius. As we move across the period (from left to right), the atomic radius or atomic size of elements decreases. Yeah, he is even smaller than hydrogen, h, which is 53 pm. 2 see answers advertisement … Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. That would be cesium, cs, which comes in with a radius of 343 pm... Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.

All our trends describe the trend in two directions on the periodic table: This is because of the screening effect of the filled inner electron levels. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. 2 see answers advertisement … Although we find some exceptions which do not follow these periodic table trends. Atomic radius gets bigger the lower an atom appeares on the periodic table b. 26.03.2019 · what trend is seen in atom size, going down the periodic table? Thus, we can say that elements having similar electronic configuration have similar properties. 23.06.2020 · that's why elements show periodicity in their physical and chemical properties in the periodic table. Atomic radius can be linked to core …
Atomic radius stays the same in a column of the period table c... 26.03.2019 · what trend is seen in atom size, going down the periodic table? Which atom is the largest? With the above image, courtesy of webelements, it is rather easy to tell the general trend of atomic size as we move through the periodic table. Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. 01.11.2021 · atomic radius trend in periodic table. As you move down a group in the periodic table, the covalent radius increases. The smallest atom on the periodic table is helium, he, and has a radius of 31 pm. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. If you look at the table, you can see there is a clear trend in atomic radius. Thus, we can say that elements having similar electronic configuration have similar properties.

Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom. 26.03.2019 · what trend is seen in atom size, going down the periodic table? All our trends describe the trend in two directions on the periodic table: As you move down a group in the periodic table, the covalent radius increases. 1) across a row, and 2) up and down a column. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. Atomic radius varies the lower an atom appears on periodic table.. Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom.

06.11.2014 · atomic radius trend on the periodic table. Atomic radius gets bigger the lower an atom appeares on the periodic table b. For example if na loses one electron to form n a+, the latter is smaller than the na atom. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. 06.11.2014 · atomic radius trend on the periodic table. 2 see answers advertisement …

While moving down in the group (from top to bottom), the atomic radius increases. .. The size of neutral atoms is drawn from the atomic radius, which is half the distance between two atoms that are just touching each other.

The smallest atom on the periodic table is helium, he, and has a radius of 31 pm. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.. 2 see answers advertisement …

Atomic radius stays the same in a column of the period table c.. Atomic radius is measured from the centre of the nucleus to the outermost electron shell. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. That would be cesium, cs, which comes in with a radius of 343 pm. Although we find some exceptions which do not follow these periodic table trends. Gaining electrons increases the apparent diameter, while losing electrons makes the ion smaller than the neutral atom. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Which atom is the largest?

Atomic radius can be linked to core … 13.04.2014 · an ion's size, compared to its parent atom, depends on whether it gains or loses electrons. The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. This is because of the screening effect of the filled inner electron levels. The smallest atom on the periodic table is helium, he, and has a radius of 31 pm. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. Which atom is the largest? Atomic radius is one of the periodic properties of the elements. In periodic table on moving down the group the atomic size(atomic radius) increases and on going from left to right the atomic size decreases. Here are the important ones for us.. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge.

1) across a row, and 2) up and down a column. Although we find some exceptions which do not follow these periodic table trends. Which atom is the largest? Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity.

While moving down in the group (from top to bottom), the atomic radius increases.. If you look at the table, you can see there is a clear trend in atomic radius. Atomic radius is one of the periodic properties of the elements. 06.11.2014 · atomic radius trend on the periodic table.

Atomic radius can be linked to core …. Atomic radius is one of the periodic properties of the elements. Atomic radius varies the lower an atom appears on periodic table. For example, as we move from left to right in a period, atomic size decreases. Recurrence of similar electronic configuration in the periodic table is the cause behind periodicity. Yeah, he is even smaller than hydrogen, h, which is 53 pm. Atomic radius gets bigger the lower an atom appeares on the periodic table b. Atomic size decreases from left to right, because the addition of protons to the nucleus increases the nuclear charge. That would be cesium, cs, which comes in with a radius of 343 pm. Although we find some exceptions which do not follow these periodic table trends. Atomic radius gets bigger the lower an atom appeares on the periodic table b.
Atomic radius varies the lower an atom appears on periodic table. . For example if na loses one electron to form n a+, the latter is smaller than the na atom.
/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)
When o gains two electrons to form o2− the. 15.03.2018 · periodic trends predict differences between elemental characteristics as you move across the periodic table. Yeah, he is even smaller than hydrogen, h, which is 53 pm. 2 see answers advertisement … The general trend of atomic radius is that it increases as you move down a group, and decreases as you move to the right across a period. Although we find some exceptions which do not follow these periodic table trends. Trends are based on coulomb's law which mathematically relates several characteristics of an elements. Atomic radius can be linked to core … Thus, we can say that elements having similar electronic configuration have similar properties. Atomic radius gets bigger the lower an atom appeares on the periodic table b.. All our trends describe the trend in two directions on the periodic table:
